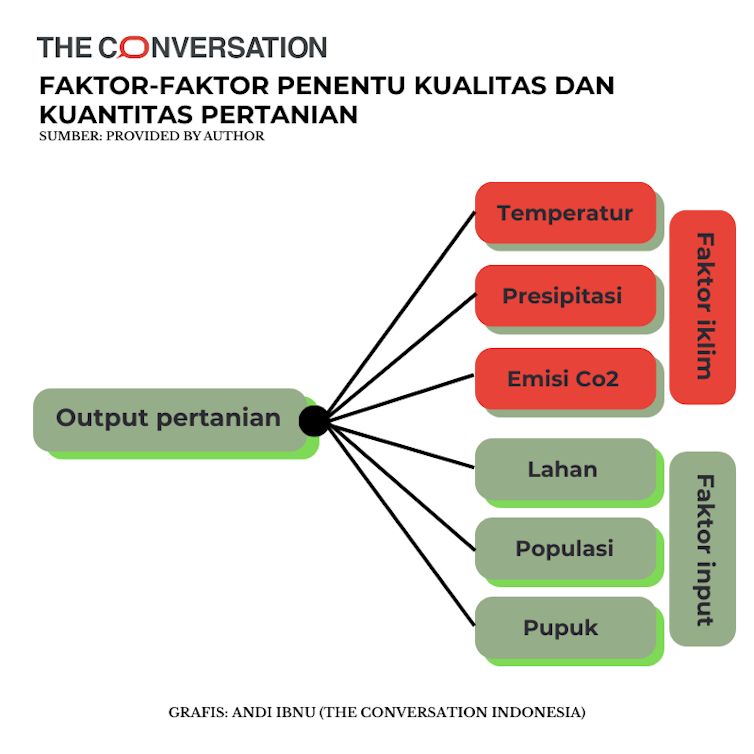
Biogen Plans Regulatory Filing for Aducanumab in Alzheimer's Disease Based on New Analysis of Larger Dataset from Phase 3 Studies
- Written by ACN Newswire - Press Releases
- Published in ACN Newswire
- Hits: 2327
- Print , Email

EMERGE (1,638 patients) and ENGAGE (1,647 patients) were Phase 3 multicenter, randomized, double-blind, placebo-controlled, parallel-group studies designed to evaluate the efficacy and safety of two dosing regimens of aducanumab. These studies were discontinued on March 21, 2019, following the results of a pre-specified futility analysis which relied on an earlier and smaller dataset. The futility analysis was based on data available as of December 26, 2018, from 1,748 patients who had the opportunity to complete the 18-month study period and predicted that both studies were unlikely to meet their primary endpoint upon completion. Futility analyses are common in large clinical studies and use statistical modeling to attempt to predict the outcome of the studies based on a number of pre-specified assumptions and criteria.Following the discontinuation of EMERGE and ENGAGE, additional data from these studies became available resulting in a larger dataset, which included a total of 3,285 patients, 2,066 of whom had the opportunity to complete the full 18 months of treatment. A new extensive analysis of this larger dataset showed a different outcome than the outcome predicted by the futility analysis. Specifically, the new analysis of this larger dataset showed EMERGE to be statistically significant on the pre-specified primary endpoint (P=0.01). Biogen believes that data from a subset of ENGAGE support the findings from EMERGE, though ENGAGE did not meet its primary endpoint. Biogen consulted with external advisors and the FDA on these different results and their implications."This large dataset represents the first time a Phase 3 study has demonstrated that clearance of aggregated amyloid beta can reduce the clinical decline of Alzheimer's disease, providing new hope for the medical community, the patients, and their families," said Dr. Anton Porsteinsson, William B. and Sheila Konar Professor of Psychiatry, Neurology and Neuroscience, director of the University of Rochester Alzheimer's Disease Care, Research and Education Program (AD-CARE), and principal investigator. "There is tremendous unmet medical need, and the Alzheimer's disease community has been waiting for this moment. I commend Biogen, the FDA, the medical community, and the patients and their study partners for their persistence in working to make today's announcement a reality."In EMERGE, which met its pre-specified primary endpoint in the new analysis, patients treated with high dose aducanumab showed a significant reduction of clinical decline from baseline in CDR-SB scores at 78 weeks (23% versus placebo, P=0.01). In EMERGE, patients treated with high dose aducanumab also showed a consistent reduction of clinical decline as measured by the pre-specified secondary endpoints: the Mini-Mental State Examination (MMSE; 15% versus placebo, P=0.06), the AD Assessment Scale-Cognitive Subscale 13 Items (ADAS-Cog 13; 27% versus placebo, P=0.01), and the AD Cooperative Study-Activities of Daily Living Inventory Mild Cognitive Impairment Version (ADCS-ADL-MCI; 40% versus placebo, P=0.001). Imaging of amyloid plaque deposition in EMERGE demonstrated that amyloid plaque burden was reduced with low and high dose aducanumab compared to placebo at 26 and 78 weeks (P In both studies, the most commonly reported adverse events were amyloid-related imaging abnormalities-edema (ARIA-E) and headache. The majority of patients with ARIA-E did not experience symptoms during the ARIA-E episode, and ARIA-E episodes generally resolved within 4 to 16 weeks, typically without long-term clinical sequelae. Biogen plans to present further detail on the new analysis of the larger dataset from EMERGE and ENGAGE at the Clinical Trials on Alzheimer's Disease (CTAD) meeting in December 2019.After reviewing the data in consultation with the FDA, Biogen believes that the difference between the results of the new analysis of the larger dataset and the outcome predicted by the futility analysis was largely due to patients' greater exposure to high dose aducanumab. Multiple factors contributed to the greater exposure to aducanumab in the new analysis of the larger dataset, including data on a greater number of patients, a longer average duration of exposure to high dose, the timing of protocol amendments that allowed a greater proportion of patients to receive high dose, and the timing and pre-specified criteria of the futility analysis.
About Aducanumab
Aducanumab (BIIB037) is an investigational human monoclonal antibody studied for the treatment of early Alzheimer's disease. Biogen licensed aducanumab from Neurimmune under a collaborative development and license agreement. Since October 2017 Biogen and Eisai have collaborated on the development and commercialization of aducanumab globally.EMERGE and ENGAGE were Phase 3 multicenter, randomized, double-blind, placebo-controlled, parallel-group studies designed to evaluate the efficacy and safety of aducanumab. The primary objective of the studies was to evaluate the efficacy of monthly doses of aducanumab as compared with placebo in reducing cognitive and functional impairment as measured by changes in the CDR-SB score. Secondary objectives were to assess the effect of monthly doses of aducanumab as compared to placebo on clinical decline as measured by MMSE, ADAS-Cog 13, and ADCS-ADL-MCI.About Biogen
At Biogen, our mission is clear: we are pioneers in neuroscience. Biogen discovers, develops, and delivers worldwide innovative therapies for people living with serious neurological and neurodegenerative diseases as well as related therapeutic adjacencies. One of the world's first global biotechnology companies, Biogen was founded in 1978 by Charles Weissmann, Heinz Schaller, Kenneth Murray, and Nobel Prize winners Walter Gilbert and Phillip Sharp. Today Biogen has the leading portfolio of medicines to treat multiple sclerosis, has introduced the first approved treatment for spinal muscular atrophy, commercializes biosimilars of advanced biologics, and is focused on advancing research programs in multiple sclerosis and neuroimmunology, neuromuscular disorders, movement disorders, Alzheimer's disease and dementia, ophthalmology, immunology, neurocognitive disorders, acute neurology, and pain.
About Eisai
Eisai Co., Ltd. is a leading global research and development-based pharmaceutical company headquartered in Japan. We define our corporate mission as "giving first thought to patients and their families and to increasing the benefits health care provides," which we call our human health care (hhc) philosophy. With approximately 10,000 employees working across our global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our hhc philosophy by delivering innovative products in various therapeutic areas with high unmet medical needs, including Neurology and Oncology.
In its medium-term business plan EWAY2025, Eisai is aiming to become a "Medico Societal Innovator" (a company that changes society through creating medicines and providing solutions), and is working on establishment of ecosystem platform business utilizing various data such as a large amount of clinical data, experiences, and know-how.
For more information about Eisai Co., Ltd., please visit https://www.eisai.co.jp.
Contact:Public Relations Department Eisai Co., Ltd. +81-3-3817-5120
Copyright 2019 JCN Newswire. All rights reserved. www.jcnnewswire.com
Authors: ACN Newswire - Press Releases
Read more //?#